Giovanni Occhipinti
Chapter 7.
The choice of the amino acids
The problem has often been mentioned: in the Miller experiment and other researchers and even in meteorites about 60 amino acids have been identified, but only 20 have become part of the proteins. So the question is: because only 20 amino acids were selected? And then, were these twenty amino acids all present in the prebiotic era?
As already illustrated, if the appliance is used to measure the flow potentials or if it is left in stand-by in contact with the reference solution, a decrease in the flow potential as a function of time occurs.
This decrease in potential does not occur continuously, but discretely with jumps of about 0,20mV. Furthermore, the potential flow of the capillary diaphragm of quartz in contact with the reference solution varies from a maximum of 10.10 mV to 7.50 mV. Below this last value the potential is not stable. The flow potentials are therefore within an interval of about 2,60mV. This means, considering as already mentioned drops of 0.20 mV, that the Quartz having 13 potentials at its disposal was able to select, i.e. adsorb on its surface, only 13 amino acids.
But as we have already seen in the paragraph on the genetic code, Leu is selected from two specific potentials: 9.50 mV and 8.10. The potentials available to the Quartz drop to 12 and therefore 12 are the amino acids that the Quartz could select.
We know, however, as can be seen from the genetic code, that seven pairs of amino acids are already coded by the first two letters, that is, in seven cases, two amino acids are coded by the same base pair in 1st and 2nd positions. They are:
(Phe, Leu) – UU∙, (Ileu, Met) –AU∙, (His, Gln) – CA∙, (Asn, Lys) –AA∙,
(Asp, Glu) – GA∙, (Cys, Trp) –UG∙, (Ser, Arg) –AG∙
Now, as we have already highlighted, the amino acids pairs available to us are selected from the same potential, in particular Phe and Leu at 9.50 mV, Ileu and Met at 9.30. But then the other amino acid pairs are also selected from only one specific potential. So for seven potentials we have 7 more amino acids which brings us to 12 + 7 = 19. Therefore, the 12 potentials available to the Quartz can select 19 amino acids. Now, if we extend the electrokinetic effects observed with quartz to colloidal silica, we can conclude that the only potentials available in nature, in the prebiotic era, were the specific potentials corresponding to the 19 amino acids contained in our proteins. Other amino acids could not be selected because there were no other potentials that could be used to select other amino acids. The missing 20th amino acid, glycine (Gly), could not be selected from colloidal silica because it does not present Right and Left, it does not present chirality. Glycine is chemically the simplest of amino acids and in Miller’s experiments, it is the most abundant. It is probable then that it entered the constitution of the primitive polypeptides due to its high concentration in the prebiotic era.
From this analysis, it emerges that the twenty amino acids that make up our proteins could be selected, in quality and quantity, by chemical-physical processes.
Remains the question: were the twenty amino acids all present in the prebiotic era?
The amino acids in question are:
Gly (glycine), Ala (alanine), Val (Valine), Leu (leucine), Ileu (isoleucine), Pro (Proline), Asp (aspartic acid), Glu (Glutamic acid), Ser (serine), Thr (threonine ), Gln (glutamine), Asn (asparagine), Met (Methionine), Cys (cysteine), Phe (phenylalanine), Tyr (Tyrosine), Trp (tryptophan), Hys (histidine), Arg (arginine), Lys (Lysine).
The greatest part of scientists, which are studied the origin of life, are of the opinion that the number of amino acids, when life began, was certainly less than today and that some amino acids were added later to proteins. Few, however, are researchers who declare the numbers and names. Among them who tackled in a comprehensive way the issue was Mario Ageno, (quoted work). He began from a Miller's work of 1974, which reviews the results of all the experiments conducted up to that time and proves, beyond any doubt, the prebiotic synthesis of 12 amino acids synthesis:
Gly, Ala, Val, Leu, Ileu, Pro, Asp, Glu, Ser, Thr, Gln, Asn
Miller shows how, adding H2S (hydrogen sulphide) to the primitive-atmosphere model (CH4, NH3, H2O, H2), is easily obtained.
Met
While the
Cys
was obtained from Khane and Sagan by exposing under ultraviolet radiation a mixture of: CH4, C2H6, NH3, H2O, H2S.
Also through pyrolysis processes and reactions in solution are obtained.
Phe, Tyr, Trp
Finally, from the synthesis of Pro and pipecolic acid they are obtained
Arg, Lys.
The Histidine is the only amino acid that has never been synthesized via prebiotic procedure.
Of course it is not excluded, in fact it is very likely, that the processes of synthesis of the amino acids in the prebiotic era, in many cases, have followed other steps than those described above.
We focus for the moment on the 12 amino acids of sure prebiotic origin and see later what other processes of abiotic synthesis of amino acids can be considered plausible.
Ageno suggests starting from a formal study of the genetic code, i.e. the law of correspondence, represented with 3:1, between amino acids and RNA.
The RNA is the nucleic acid that carries the information for the synthesis of proteins.
As we have seen, having available four nucleotides, the ways in which we can dispose them 3 by 3 are 43 = 64. Three of these triplets are used as end signal (t.), then, in theory, RNA contains the information for 61 amino acids. Since the amino acids in all living organisms are only 20, the genetic code is degenerate meaning that more triplets code for the same amino acid. For completeness, we report once again the table of the genetic code.
In relation to the amino acids present in the prebiotic era, analysing the genetic code, Mario Ageno writes: «The same formal structure of the genetic code suggests that the two amino acid methionine (Met) and tryptophan (Trp) are of fairly recent introduction.
He then takes into account the studies on the amino acid sequence of proteins, in particular haemoglobin and cytochrome, contained in different species. He analyses a particular type of protein considered of ancient origins: the Ferredoxins. As known, one of the evolutionary processes that leads to new proteins is the doubling of the gene. This gene will express a double-length protein but consists of two identical amino acid sequences that with time, due to the effect of the mutations they will diverge. However, it is often possible to go back to the original protein, and it is what has been done with Ferredoxins. It can be shown that these proteins derive all from an original protein of 27 amino acids, considered one of the first proteins appeared during the process that gave rise to life.
This ancient protein is made up of nine different amino acids that are:
Gly, Ala, Val, Glu, Asp, Pro, Cys, Ser, Ileu
Following a different reasoning, Jukes also adds Leu. As you see, all except Cys, are part of the 12 amino acids, of sure prebiotic origin by Miller's review. Ageno then suggests, that the number of amino acids in the prebiotic period could be about half of the current ones.
This conclusion, however, creates problems.
If in the prebiotic era only 10 amino acids were present, many triplets would be meaningless. For example, four triplets with C and G in the 1st and 2nd position and two triplets with A and G in the 1st and 2nd position all encode Arg. Since Arg is not among the ten listed above, and not even among the 12 of Miller's review, these triplets would be meaningless triplets. But Ageno categorically rules out the existence of meaningless triplets in the prebiotic era, because isolated mutations would have disrupted protein synthesis with lethal results. To solve the problem, he introduces the "wobbling" phenomenon. He imagine that the central letter -U- indicate a hydrophobic amino acid (which does not bind with H2O) while -A- indicate a hydrophilic amino acid (which binds with H2O), leaving the precise specification of the results to evolution. But if you leave the choice to future evolution, another problem is created; each species would have chosen its own specification and the genetic code would not be universal. This explanation, however, creates other problems. Why Arg and Ser despite being hydrophilic not have A in second position but C? And why doesn’t the Pro have no U in second place despite being a hydrophobic? Yet in both first and second case there should be triplets available because, as seen, more triplets encode the same amino acid, for example the Pro could have replaced one of the Leu triplets. It should also be added that the appearance of a new amino acid also results in the opening of a new metabolic pathway. It is quite difficult to imagine how life, newly formed and evolving, can resist the stress of constantly creating such a large number of new metabolic pathways.
We can conclude that the problem of how many amino acids there were in the prebiotic era, which ones and how they were chosen is still unresolved until here.
We then resume Miller's work. As seen, both among the 12 amino acids of the review, among the components of the ancient protein, Pro is present. But as Ageno suggests the synthesis of Pro involves the synthesis of Arg and Lys. But the Arg could not be missing, otherwise we would have had 6 nonsense triplets. It seems quite reasonable to add to the list of 12 also these two amino acids. So 14 turn out the amino acids present in prebiotic era.
Let us now consider the synthesis of Met and Cys.
As we have seen in the review of Miller, adding H2S (hydrogen sulphide) to the primitive atmosphere model (CH4, NH3, H2O, H2) you get the Met, while the Cys was obtained from Khane and Sagan under ultraviolet radiation of a mixture of: CH4, C2H6, NH3, H2O, H2S. Now, at first glance the formation of Met seems chemically simpler than Cys. Yet the Cys is one of nine amino acid components of the Ferredoxins, ancient proteins appeared during the process that gave rise to life. But, if Cys formed, definitely also Met had to be formed. We actually have no valid element that can do us to exclude the Met. Even these two amino acids should therefore be added to the twelve already listed. However, since no researcher has ever suggested that the earth's atmosphere contained H2S at the global level, we must assume that these two amino acids are formed only in places where H2S was present that is in proximity to volcanic areas.
According to Christian de Duve (quoted work, 2008), Tryptophan (Trp), and Histidine (Hys) appeared later in the development of life. In fact, they have not been found in meteorites and also Histidine has never been synthesized in prebiotic chemistry experiments. Recalling again Miller’s experiment, Phe (Phenylalanine), Tyr (Tyrosine), Trp (Tryptophan) were obtained through pyrolysis processes and reactions in solution. The pyrolysis processes, i.e. demolition of compounds by heat, are processes that occur at high temperature. In the laboratory we get good amounts as we have well-controlled processes. These three amino acids have a relatively complex molecular structure and the Trp is the most complex of the three. But de Duve does not exclude the Trp for the complexity of its molecule but only because it was not identified in meteorites. This does not seems a discriminating reason. And so, either we exclude all three of them, or we imagine that, in the prebiotic era, modest amounts of these compounds were formed in particular areas of the earth's crust, for example in the presence of cooling lava.
The analysis done until now it suggest two conclusions.
A) The amino acids present in prebiotic era were 14:
Gly, Ala, Val, Leu, Ileu, Pro, Asp, Glu, Ser, Thr, Gln, Asn, Arg, Lys
The other 6 were absent.
B) The 20 existing amino acids were all present but the first group, spread over the entire surface of the planet, consists of 14 amino acids listed from above:
Gly, Ala, Val, Leu, Ileu, Pro, Asp, Glu, Ser, Thr, Gln, Asn, Arg, Lys
A second localized group, meaning that they formed in particular areas of the planet probably in the proximity of volcanic areas, consists of 6 amino acids.
Met, Cys, Phe, Tyr, Trp, Hys
Now, let us follow a different argument.
As we have already said (chapter 6), Ageno does not elaborate on the consequences of the fact that the pair of letters in the 1st and 2nd position were read.
And then, we start from the structure of the genetic code and in particular by a necessary condition: there can’t be meaningless triplets. This condition can be satisfied if the 1st and 2nd position are used for encoding at least one amino acid and the third position as spacing. This possibility has already been admitted by Ageno when he writes: «However, at the beginning not all three positions were read: maybe the first two and the third had the spacing function». Now, if the third position is reduced to spacing, the number of amino acids that the genetic code can encode using only the 1st and the 2nd position is reduced to 24 = 16. This means that in the prebiotic era we should have 16 amino acids, otherwise we would have been in the presence of meaningless triplets.
By the genetic code, however, Ser and Arg occupy the triplets in the
1st and 2nd position CC and CG respectively and the triplets AG in 1st and 2nd position. In short, two amino acids occupy three positions and so to not have meaningless triplets 15 amino acids are sufficient.
Furthermore, also the Leu is encoded by triplets in 1st and 2nd position, CU and UU. The Leu occupies 2 positions alone and the necessary amino acids goes down to 14.
In conclusion, in order not to have meaningless triplets, 14 amino
acids were necessary.
We are still missing 6 amino acids.
Meanwhile, it is remarkable that these numbers coincide exactly with the conclusions on Miller’s review reported in A) and B).
Let us follow the structure of the genetic code and try to identify any 6 missing amino acids.
As can be seen from the genetic code, there are pairs of amino acids that are encoded by the same nucleotide pairs in 1st and 2nd position, they are:
UU- (Phe, Leu), AU-(Ileu, Met), CA-(His, Gln), AA- (Asn, Lys), GA- (Asp, Glu), UG- (Cys, Trp), AG- (Ser, Arg)
Furthermore UA- (Tyr, t.), were t. the end signal.
In order not to have meaningless triplets, in the prebiotic era, at least one of these amino acids had to be present.
Meanwhile Asp and Glu were certainly present because they have both been identified in ancient proteins and by amino acids of certain prebiotic origin as in Miller’s review.
Even Ser and Arg had to be present otherwise, as we have mentioned before, we would have meaningless triplets.
Finally Leu, Ileu, Gln, Asn, Cys had to be present because they were already identified either among the sure amino acids of prebiotic origins or in the ancient origins of proteins.
The 6 missing amino acids would then be:
Met, Phe, Tyr, Trp, His, Lys.
With Lys in place of Cys these amino acids correspond exactly to the 6 amino acids group which, as we have assumed in B), probably they were formed in volcanic areas. It is important here to emphasize that, following two completely different paths, less than one amino acid, we obtained the same results both in quantity and in quality.
So were these 6 amino acids really missing?
For Met, Phe, Tyr, Trp is the same logic that brought us to conclusions A) and B).
Arg (Arginine) and Lys (Lysine) are two molecules with basic side chain, but the molecule of Lys is simpler than the Arg molecule. Therefore, it is not logical to exclude Lys.
Do we really want to exclude His just because it was not detected in the prebiotic experiments?
Then how many and what were the amino acids in the prebiotic era?
To conclude we start from some considerations of Ageno. He begins by noting that the introduction of a new amino acid is not the result of a simple mutation but requires the setting of a new metabolic pathway. Also, if the introduction of new amino acids were easy, why is the genetic code universal? And how the different species did not develop their own particular code?
Ageno admits he does not know the answer to these questions.
Actually, an answer to these questions can be given, and the conclusion is already exposed in B):
The current 20 amino acids were all present but are divided into two groups. A first group, spread over the entire surface of the planet, were formed from amino acids considered, by Miller, for sure abiotic origin, to which Arg and Lys must be added, in total 14, that is:
Gly, Ala, Val, Leu, Ileu, Pro, Asp, Glu, Ser, Thr, Gln, Asn, Arg, Lys
A second localized group, which is formed in particular areas of the planet probably near volcanic areas, consisting of 6 amino acids.
Met, Cys, Phe, Tyr, Trp, Hys
This is, in short, an almost obligatory conclusion and coincides with that of the first analysis based on the specific potentials of amino acids. If the genetic code is universal, it follows that it originated when life originated, then the 20 amino acids were all be present. For life in formation, setting up new metabolic pathways each time the appearance of new amino acids and therefore continually changing the genetic code would have been lethal. All this however leads us to conclude that the proteins that were formed near volcanic areas had to contain the amino acids that were formed in these areas and consequently future cells develop different metabolic pathways. We must then clarify, and we will do it later, because life is unitary.
Some researchers wondered why exactly these 20 amino acids and not others?
The matter is dealt by Arthur Weber and Stanley Miller in "Reasons of the Occurrence of the Twenty Coded Protein Amino Acids" in 1981. They report the works of various researchers, for us of no interest, because they start from the assumption that life has its origin in a prebiotic soup. Moreover, the two scientists claim unlikely that a single process can explain the selection of the twenty amino acids.
Here we argues that the prebiotic soup has never existed and that life originated on firm land. We also argue that the experiment on electrical double layers is still the only known experiment, of a single process, where a physical agent, quartz, deductively connects the separation, in prebiotic times, of the amino acids Right from Left with the principles fundamentals of physic.
We assume, as we have already explained in the previous chapters, that the colloidal silica has the same behaviour of quartz.
Since each amino acid has an electromagnetic field associated with its molecule, of about 60 amino acids present in the prebiotic era, 19 amino acids were chosen because their electromagnetic field was compatible with the electrokinetic potentials of colloidal silica, the 20th, Glycine due to its high concentration.
Chapter 8.
The synthesis of polypeptides
8.1 The problem of water in living organisms
Let us imagine a full water bottle and you place it upside down in a container, which is also full of water. No one, not even those who have never done this experience as a kid, would expect the bottle to get empty. Although it is a bit obvious, it seems an appropriate metaphor to clarify the problem of the synthesis of the fundamental macromolecules of life.
The reaction of hundreds of peptide bonds for the formation of proteins,
The reaction for the formation of the nucleotides constituent of nucleic acids,
and the thousands of bonds for the formation of nucleic acids,
The reaction for the formation of sugars
and lipids
they also give water as a reaction product
These reactions are actually equilibrium reactions, i.e. reactions where the products of the reaction, after reaching a certain concentration, always remain constant; further formation of products decompose in order to restore the reactants. Reactions of this type are represented by a double arrow and, in the case of peptides, we simplify them in the synthesis of a dipeptide, in which Ala indicates the amino acid Alanine and Gly the Glycine.
Ala+Gly ⇄ Ala - Gly + H2O
In aqueous medium, and then in a prebiotic soup, these synthesis reactions cannot occur because the abundance of water already present, pushes the reaction to the left. In a simplistic way, as the water cannot come out spontaneously from the bottle, if also immersed in a container filled with water, so, in an aqueous environment, the water cannot get out, of the reactions mentioned above, spontaneously. And if the water is not produced, the synthesis of the macromolecules essential for life does not take place.
Now the question is that all the cells living organisms contain water and within them these reactions take place. How is it possible?
Let us return to the metaphor of the water bottle and reactions.
If, keeping the bottle upside down, we want to get the water out, we have to take it out of the container, that is, we have to do work, we need to provide energy. Similarly, in living organisms, in order to let water out of the synthetic reactions listed above, it is necessary to supply energy. Part of this energy is supplied by catalysts, enzymes, which lower the energy necessary for the reactions; the rest of the energy is supplied by molecules that act as "fuel" by taking away the water.
In many cases, the enzymes create conditions that do not require “fuel” molecules. As reported by Pier Luigi Luisi (quoted work) «The large size of the enzymes are necessary because in the proximity of the active site a non-aqueous microenvironment forms. These conditions allow the enzyme an extraordinary reactivity which is different from reactions than in an aqueous environment».
Finally, in living organisms, newly formed macromolecules are immersed in aqueous solutions where they are unstable. To avoid the disruption they take on particular structures.
In conclusion, in all the living organisms the synthesis of macromolecules reactions require a: 1) catalysts, 2) non-aqueous microenvironments 3) some source of energy 4) a mechanism that stabilizes macromolecules.
8.2 The problem of water in the prebiotic era
Vital processes must have needed a necessary continuity with the processes that led to the origin of life. Hence, the same tools needed today for living organisms, must surely have been necessary in prebiotic times for the synthesis of the fundamental macromolecules of life.
Since, as we have said, these are equilibrium reactions, we start from the study of these rations.
It is challenging the fact that one comes to the same conclusion starting from another perspective.
The substances in the liquid state, gaseous and in solution move and this movement depends on the temperature. The branch of chemistry that studies the movement of particles and deals with the mechanism of the reactions, as we have already highlighted, is called chemical kinetics.
The chemical kinetics states that: necessary and sufficient condition for a reaction to occur is that there is a collision and that the collision is effective. A certain energy is therefore necessary, called activation energy E1, to break the bonds that already exist in the reactants. This statement can be represented graphically by placing on the vertical axis the energy and the horizontal axis the reaction coordinate that indicates the course of the reaction.
For the reaction of formation of the proteins that we schematized in the equilibrium reaction of the dipeptide, Gly + Ala ⇄ Ala - Gly + H2O, X represents the reactants (amino acids) and Y the compounds (peptides).
This chart takes us step by step to the understand of how the formation reaction of the peptides in the prebiotic era, could occur. In particular:
1) Going in search of minerals in the prebiotic era which functioned as a catalysts and lowered ( from E1 to E2) the energy needed for the reaction to take place.
2) Looking for conditions that have further reduced (from E2 to E3) the energy required for the reaction, that is non-aqueous microenvironments.
3) Finding a source of energy to overcome the remaining energy barrier and getting the products.
4) E4 indicates that the products need little energy to restore the reagents. Then find a mechanism that stabilizes the macromolecules, i.e. the products of the reaction and brings the energy necessary for the decomposition from E4 to E5.
We have come to exactly the same conclusions we got analyzing the reactions of formation of macromolecules, which occur in living organisms. So, in order to understand how the formation of macromolecules occurred in the prebiotic era, we must go in search of:
1) catalysts, 2) non-aqueous microenvironments, 3) some energy source, 4) a mechanism that stabilizes macromolecules.
So let us try to give an answer to these 4 points.
1) The search for the catalysts.
The search for inorganic catalysts that could have helped, in the prebiotic era, the formation of proteins has involved a considerable number of scientists. Since silicon is the most common element of the Earth's crust and silicates cover more than 90% of the crust, the research is directed towards these minerals and, in particular, to the components of their disintegration: the clays.
Many researchers have shown that it is possible to obtain polypeptides using the amino acids in the presence of clay.
As reported by Graham Cairns-Smith in “Argille e origine della vita: frontiere della vita”, 1998, M. Paecht-Horowitz and other colleagues (1970) were able to polymerize activated amino acids in the presence of montmorillonite. N. Lahav, D. White and S. Chang (1978) were able to assemble molecules of glycine in the presence of montmorillonite, through a cycle in which alternated absence and presence of water. Even Bujdak et al. 1995 obtained similar results. Cairns-Smith adds, presumably, polymerization is favoured by the absence of water. In fact, in the experiment of Lahav and Chang the temperature bounced between 25 to 95 degrees.
We remind that the clays contain colloidal silica and amorphous silica (silica gel) and as reported by Antonio Cadeddu in "Genesi di una teoria scientifica" in 1998: «In the midst of other physical-chemical factors acting on the enzymatic processes, we should give special attention to the structure of colloidal formations. [...] According Smuk, also inorganic gels with their very elementary structure can influence the direction of the catalytic reactions. Thus, for example, the addition of silica to an aqueous solution of acetic acid and methyl alcohol gel facilitates the catalytic synthesis of the ester”. Note that in this reaction one of the products is just water, such as in protein synthesis. On the other hand, the use of the silica gel in the industry is well known both, as catalyst and as a dehydrating agent.
And so, if it is possible that the clays have catalysed the protein synthesis reaction, in the prebiotic era, why is the matter not considered closed?
The problem is that the clays:
A) They are not selective in the sense that they do not recognize amino Right acids from Left and therefore they catalyse the two forms.
B) Do not recognize the 20 biological amino acids among the approximately 60 present in the prebiotic era and catalyse any type of amino acid, giving rise to any polymer. But the chemical evolution requires particular polymers to perform specific functions within the proto organisms. Ultimately, the presence of clay and / or amorphous silica catalyses generically the bond between amino acids, i.e. the peptide bond:
R'COOH + R "NH2 ⇄ R'C=O-NH-R''+ H2O
The only selective mineral in relation to amino acids, as we have seen elsewhere, is the silica Sol, the colloidal silica. The explanation for this diversity has been deeply illustrated: solutions in contact with surfaces give rise to double electrical layers. In the presence of clays, the electric field inside the double layer has parallel and equidistant lines of force, which means, the field is uniform. Since the amino acid molecules have helical dipoles, they cannot enter inside the electrical double layer of the clay. Instead, the electrical double layer that is generated in contact with the colloidal silica has an internal electric field with helical lines of force where the amino acids may accumulate.
The catalytic action of the clay acts from a distance on all the amino acids, while that of the colloidal silica takes place inside of the electrical double layer and is selective.
Conclusion: the clays are good catalysts, but the colloidal silica is the only mineral that can function as selective catalyst.
2) The search for non-aqueous microenvironments.
Let’s go back to the reaction Ala +Gly ⇄ Ala - Gly + H2O and imagine to have 100 molecules of Ala and 100 Gly molecules that after an hour have reached equilibrium resulting in 10 molecules of Ala-Gly and 10 water molecules (quantity and time are chosen randomly). These quantities, in time, do not vary anymore, they remain constant. If the reaction is carried out in the presence of a catalyst, instead of waiting for an hour equilibrium is reached in a few minutes. That is, 10 Ala-Gly and 10 water molecules will always be formed, which will remain constant over time. Therefore, the catalyst accelerates the reaction but does not move the chemical equilibrium.
How do you shift the equation to the right?
There exists a chemical principle that in our case one could synthesize like this: remove the water and the equilibrium moves to the right, towards the formation of the dipeptide. Which is like saying: empty the container and the water will come out spontaneously from the bottle.
The problem of how to eliminate or reduce the water and therefore its action on the synthesis reactions has always been associated to heat. In 1963, Sidney Fox warmed an amino acid solution to a temperature of 130°C to obtain a mixture of polymers that has called "proteinoid". The heat has in this case two functions: remove the water favouring the reaction, increase the thermal agitation of the amino acid molecules in order to have an effective impact. As we have seen above, even in the presence of clays as catalysts, the heat that is supplied retains the same purpose.
But can the heat really be used to remove water and provide energy for the synthesis reactions?
In 1978, Richard E. Dickerson (cited article) made a proposal: The evaporation caused by the sun, in a freshwater pond, it may have produced the synthesis of macromolecules; but then he adds: «One objection to this proposal is that several of the important precursors of biological molecules, such as hydrogen cyanide, cyanogen, formaldehyde, acetaldehyde and ammonia are themselves volatile». Note that these substances are precursors of the Ribose and the nucleobases that constitute nucleic acids. Therefore, if one removes the water through heating one also eliminates the precursors of the nucleic acids and thus blocks the formation of the basic substances required for the origin of life, which is like cutting the branch on which one sitting on. In conclusion, we cannot use heat to remove the water but we must create dry compartments in aqueous solutions.
These compartments exist and are found in aqueous solutions in contact with the various clay components; they are the double electrical layers similar to micro capacitors, which we have extensively discussed. As we remember Giuseppe Bianchi in (quoted work) 1963 «[...], have a tendency to be expelled from the electrical double layer species with lower dielectric constant to be replaced from species to higher dielectric constant» Amino acids have a high dielectric constant and helical dipoles. They can then enter the electrical double layer of the colloidal silica expelling the water molecules. In the absence of water, the equilibrium is shifted to the right, towards the formation of peptides.
Conclusion: inside of aqueous solutions in contact with the colloidal silica contained in clays, are formed microenvironments non-aqueous, as occurs in living organisms near the enzymes. The balance Ala + Gly ⇄ Ala-Gly + H2O, the water removed is moved to the right, towards the formation of peptides.
3) The search for a source of energy.
Not being able to use the heat as an energy source for the synthesis of polypeptides, many scientists went looking for energy-rich molecules such as HCN or inorganic pyrophosphate that, in the prebiotic soup, functioned as "fuel".
As reported by R. F. Doolittle and P. Bork in “La modularità delle proteine" Le Scienze Quaderni 1996, around 1970 M. G. Rossmann proposed that proteins were made up of modules (domains), which appeared early in the history of life and assembled in different combinations. This hypothesis was confirmed. Many proteins are made up of modules (or domains) with a number of amino acid residues between 45 and 70. As we have seen elsewhere, the Ferredoxins all derive from a primordial protein of 27 amino acids, considered one of the first proteins to have appeared during the process that gave rise to life. Today it is sure that when a polypeptide chain contains 30-40 amino acid residues (some authors will push to 20 amino acids) begin to have cohesive forces sufficient to assume a predominant form. So, we will use a polypeptide of 30 amino acids as the reference and see how to synthesize them in prebiotic soup.
The HCN (hydrogen cyanide) is an energy-rich molecule and was present in the prebiotic era. The reaction of the formation of a polypeptide with 30 amino acids should follow this path. A molecule of amino acid would have to initially react with HCN and subsequently with a second amino acid molecule obtaining a dipeptide. The dipeptide obtained would have to again react with HCN, and then with another amino acid molecule obtaining a tripeptide, and so on for 30 molecules. Reactions of this type are thermodynamically possible; they have a 100% efficiency. For the formation of a single peptide bond, however, it takes at least five intermediate reactions. The synthesis, by this way, of a polypeptide of 30 amino acids, required that, in the primordial broth, about 150 consecutive chemical reactions for a single polypeptide molecule take place by chance.
The primordial broth brings prebiotic chemistry to evanescence.
To clarify the question of the source of energy it is perhaps appropriate to review some thermodynamic criteria and abandon the idea of prebiotic broth.
Let us imagine we have a beaker containing 100 grams of water and add a bit of alcohol. The thermodynamics provides us with the analytical tools to demonstrate that the ethyl alcohol is miscible with water.
Now let us imagine a glass surface where we deposit a drop of water. Close to the water, we deposit a drop of ethyl alcohol. The drop of expanding alcohol is approaches the drop of water. Since the two substances are miscible, we expect that as soon as the alcohol reaches the drop of water they mix.
However, as the drop of expanding alcohol approaches the water, this moves away continuously; water and alcohol do not mix; it is exactly the opposite of what we expected according to thermodynamics.
Let us take another example. In a beaker containing 100 grams of water, we add a bit of H2SO4 (sulfuric acid). The thermodynamics states: the H2SO4 can be mixed with water and in addition, heat is produced.
Now we place four water drops on the sides of an imaginary square on a glass surface.
At the centre of the four drops, we place micro drops of sulfuric acid.
The latter slowly expand. Since the two substances are miscible, we expect that as soon as the sulfuric acid reaches the drops of water they mix. Well, also in this case the sulfuric acid does not mix with water but tries to find an escape route too. The opposite of what thermodynamics provides. Do not try to replace ethyl alcohol with concentrated nitric acid (HNO3), you might be reminded of the existence of a "purpose" (Appendix 1).
Why do these experiments run on the contrary to the thermodynamics?
The reason is the following: when a reaction is made to take place in a beaker, both walls of the beaker and that of the air above (i.e. the set of the contour) have an influence on the process. Moreover, we took a quantity of water such that the effect of the contour on the process is practically negligible. These are the processes that the thermodynamics studies, processes involving large masses, large-scale processes, where the contour is negligible. The drops are small masses where the outline is predominant, in which the glass-alcohol interaction (or sulfuric acid), and air-alcohol (or sulfuric acid), and the glass-water and air-water interaction predominate. This type of thermodynamics, which we can define on small scales, has never been studied and we do not know how to study it. As these experiments show, thermodynamics at small scales can move spontaneously in the opposite direction compared to the same thermodynamics at large scales.
Hence, small scales dynamics cannot be explained by classical thermodynamics.
There are researches, which states that at small scales (nanoscales) the second law of thermodynamics, for a short time, can be neglected, or that, at the nanoscale, the laws we studied in school no longer apply. I do not have the competence to enter into the merits of this research. Here we want to state that the second law of thermodynamics is always valid. It, however, at small scales, does not provide appropriate analytical tools. Since we lack the appropriate analytical tools, what we can do is follow a thermodynamically credible logical thought.
But how can thermodynamics at small scales help us understand the origin of the polypeptide?
And then, is there really a need for a source of energy for the synthesis of polypeptides?
Let us imagine, in the prebiotic era, a heterogeneous solution of clay where colloidal silica and amino acids are present. In an aqueous solution pH around 7, the amino acids are in the form of dipolar ion +NH3-CHR-COO-. If two molecules of amino acids are located next to each other, they are oriented with the positive charge towards the negative and give rise to ammonium salts R'-COO- NH3+ -R. This type of orientation, energetically more stable, is that necessary for the formation of peptide bonds. As we have suggested elsewhere, the colloidal silica retains on its surface the Left amino acids, letting the Right amino acids be carried away by the water. The Left amino acids accumulate inside the electric double layer of colloidal silica and expel H2O. They then, as already suggested, are located one next to the other, adsorbed according to their specific electrokinetic potential and certainly oriented with positive charges towards negative charges, that is, towards the formation of peptide bonds.
The double electrical layers are in equilibrium with the solution, but at the same time represent separate compartments from the solution.
How much is the number of different amino acid molecules adsorbed on the surface, is difficult to imagine, tens, maybe hundreds. We take as plausible the 30 molecules.
We therefore find ourselves having amino acids in solution and groups of 30 amino acids inside the double electrical layers of the colloidal silica. Within these microcondensers the amino acid molecules are found:
a) Next to each other in a chain of ammonium salts.
b) The bonds of the functional groups are weaker because they are subject to interactions with polar covalent bonds of the colloidal silica. The silica ultimately acts as a catalyst, by lowering the activation energy.
c) The water, within these compartments, has been expelled from the amino acids and the equilibrium is shifted towards the formation of the products.
Ultimately, the energy needed for protein synthesis is reduced to a minimum and the impact between the amino acids caused by thermal agitation is already sufficient.
With the formation of a polypeptide of 30 amino acids 29 water molecules are formed. These molecules, freed in a poor water environment if not dry, find space where to move by increasing the entropy of the system and promoting the synthesis of the polypeptide.
The addition of energy is not necessary, as the thermal agitation is already sufficient. The spontaneity of the process is provided by the increase of entropy.
Conclusion: within the double electrical layers, it is possible that thermodynamics at small scales has worked, whereby the formation of the polypeptide becomes a spontaneous process. No energy input is required, the thermal agitation is enough and the spontaneity of the process is provided by the increase of entropy. The synthesis of polypeptides is therefore a spontaneous process with an increase of entropy: Chaos from order
4) The search for a polypeptide stabilization mechanism.
The colloidal silica particles have a very short life. If a colloidal silica particle, which has synthesized a polypeptide on its surface, meets other of colloidal silica particles, it will form amorphous silica. The electrical interactions between colloidal silica particles are so strong they deform each other, in fact the amorphous silica does not deviate the plane of polarized light. The polypeptide, not finding the electrical interactions, detaches from the surface and goes into solution.
Since the colloidal silica was helical, the trend of the polypeptides will also necessarily be helical
Conclusion: The formation of the more ordered and stable structure of the α-helix stabilizes even more the polypeptide because it increases the universal entropy: Chaos from order.
Finally, following the chart, we represent the four points and their answers.
1) Go in search of minerals in the prebiotic era, which may have functioned as catalysts and lowered (from E1 to E2) the energy needed for the reaction to take place.
The colloidal silica is the only mineral that can function as selective catalyst.
2) Look for conditions that have further reduced the activation energy (from E2 to E3), ultimately, dehydrated environments or where the action of water on the reaction is much reduced.
Inside of aqueous solutions in contact with the colloidal silica contained in clays, they are formed microenvironments non-aqueous, as occurs in living organisms near the enzymes. The balance Ala + Gly ⇄ Ala-Gly + H2O, the water removed, is moved to the right, towards the formation of peptides.
3) Find a source of energy to overcome the remaining energy barrier and get the products.
Within the double electrical layers, it possible that a small scale thermodynamics may have worked, where the formation of the polypeptide becomes a spontaneous process. No energy input is necessary, the thermal agitation is already sufficient and the spontaneity of the process is provided by the increase in entropy. The polypeptide synthesis is therefore a spontaneous process due to increasing entropy:
4) E4 indicates that products require little energy to return to reagents. It is therefore necessary to find a mechanism that stabilizes the macromolecules, i.e. the products of the reaction and brings the energy necessary for the decomposition from E4 to E5.
The formation of the more ordered and stable structure dell'α-helix, more stabilizes the polypeptide because the universal entropy increases and then, again: Chaos from order.
The "Arrow of time" carries with it the synthesis of polypeptides.
Chapter 9.
The origin of life: the proto-organism
9.1 Definition of proto-organism
Research on the origin of life began only in the first half of the last century, after centuries of theological and philosophical discussions. Today, after more than half a century of research, scientists have only one certainty: life originated from inanimate matter.
Then, if the question is: how did life originate? We must start from life to understand how it may have originated.
We have defined the bacterial cell as the smallest vital entity, the first stage of life.
But all scientists agree that life cannot have started directly with the cell. Even bacterial cells in their simplicity, compared to the cells of higher organisms, are however of enormous complexity. It is therefore believed that between the chemical period, that is, the phase in which the fundamental substances of life accumulated and interacted, and the appearance of the cell, there was something intermediate that scientists call: proto-organism, or proto-cell or pre-cell phase. The term proto-organism used by Mario Ageno at the beginning of the 1980s is used here, because it seems to give the idea of this intermediate stage better.
Now, the problem is that all scientists talk about proto-organism but nobody knows what it is.
The problem therefore arises, first of all to define the proto-organism, to understand how it may have originated from inanimate matter and, afterwards, how it turned into cell.
Before we go ahead, some clarification is needed.
Firstly, for proto-organism, we do not refer to a single and solitary entity that appeared on earth and from which only one cell emerged. With Proto-organism, we mean billions of billions of entities, chemically quite similar, spread across the surface of the planet. Perhaps for some researchers this is not so, anyway this is how we are considered in this discussion.
Secondly, the Proto-organism was a dynamic entity within which a chemical evolution took place, that is, processes that led billions of them to achieve the cellular state. Other proto-organisms, perhaps the majority, took the wrong path, they disappeared into the environment.
Finally, we do not know the processes within the proto-organism, we can only make hypotheses. In order to make some assumptions we must try to identify the chemical bases of the proto-organism, that is, the molecules that may have begun these processes.
Here arise another problem, scientists not only do not have a definition of proto-organism, but they don't agree how to identify the chemical bases of the proto-organism. In fact, for some scientists first compartments appeared, lipid vesicles, liposome-like membranes, within proteins and nucleic acids accumulated, capable respectively of metabolism and replication. Pier Luigi Luisi is an authoritative exponent of “compartmentalists” and as he states (cited work), proto-cell compartments are indispensable for the origin of life.
For many other scientists everything started through only metabolic processes within polymer membranes (first the metabolism), while for others only replicative molecules appeared first (replication first, RNA World).
But on these theories, Luisi writes: «All share a major problem: each of these theories must start from a series of more or less arbitrary assumptions». In particular, the “compartmentalists” world has to assume that macromolecules (nucleic acids and enzymes) are already present in the prebiotic environment, the metabolic approach instead starts from the assumption that only enzymes were already present, the RNA world starts from the assumption that a self-replicating RNA was available.
As we have repeatedly pointed out, making use of arbitrary assumption is unlikely to be a step forward. So, in order to define a sustainable definition of proto-organism, instead of starting from arbitrary assumptions, we must start from certain data and possible scenarios in prebiotic era.
If the bacterial cell is the smallest vital entity, in order to identify the chemical bases of the proto-organism, can we start asking a question: Which and how many macromolecules does a cell need to be considered living?
The bacterial cell is the smallest vital entity. Bacterial cells, however, do not all have the same number of macromolecules. The DNA of Escherichia coli, for example, has about 3,000 genes and therefore as many possible proteins, while Pelagibacter Ubique contains about 1300 of them. The smallest living bacterium known to date is an obliged parasite, Mycoplasma Genitalium which contains about 500 genes and as many possible proteins. Compartimentalists, supporters of the "RNA World" or metabolism, are convinced that a cell can live with a lower number of genes, and agree with a minimum genome of about 200 genes and as many proteins.
If this could have been the content of the minimal cell, if the proto organism was something intermediate between the chemical period and the appearance of the cell, how could the proto-organism differentiate itself from the minimal cell?
Let us then take up the definition of life: metabolism, reproduction, evolution.
Which of these three properties belonged to the proto-organism?
Evolution is a characteristic of living organisms. So in order to have evolution we must have at least the minimum living organism that is the cell. We, however, do not have the cell, but the proto-organism, and since it is not yet a living organism, evolution was absent. The proto-organism therefore had to be made up of metabolism and reproduction. The point is that evolution comes from reproduction, if there is no evolution it is because there is no reproduction and therefore the proto-organism could not contain the reproduction but only metabolism.
But can metabolism and reproduction be separated in a proto-organism?
According to Mario Ageno, (cited work) a system of only metabolism would have no biological significance because it would soon be dissipated in the environment without leaving any inheritance or trace. According to Ageno, the ability to reproduce is an indispensable feature for any system. On the other hand, as Dorothy Crawford explains about Viruses (cited work), there can be no reproduction without metabolism. Viruses are reproduced by exploiting host cell metabolism and if they do not find a host cell, they decompose.
So metabolism and reproduction cannot be separated, but proto-organism cannot contain reproduction.
How do we get out of this dilemma?
The problem, as is often the case, is a terminology issue. The reproduction term contains the replication term, that is, a cell before reproduction must replicate its genome. Living organisms reproduce, molecules replicate. The Proto-organism is not a living organism and therefore does not reproduce. The term reproduction is often used in a general sense, also for viruses. In reality, viruses within the cell do not reproduce but are replicated, i.e. they use the metabolic apparatus to replicate their own genome and increase the number until they stifle the cell. Hence, the proto-organism could not be a metabolic-reproductive system because reproduction is a feature of life, but it must have been a metabolic-replicative system. In other words, the proto-organism in order to survive only need, through a rudimentary metabolism, to replicate the damaged molecules.
So, if the proto-organism was a metabolic-replicative system, did it still needed a genome of 200 genes, needed for the minimum cell?
In summary, one is to look at which features of present living organisms may differ or be simpler in the proto-organism. Since there are different opinions on these topics, among several options, following Ockham's razor, we must choose the simplest and most credible one. We recall that William Ockham was a Franciscan Friar of the 14th century, and the Occam Razor principle is traced back to him: one must always start from simple, obvious assumptions and then add complexity if necessary. Certainly, it is not a universal principle but in our case, it may be useful.
1) All researchers believe that the proto-organism originated in closed compartments. As we have already explained, some scientists believe that these compartments were polymeric membranes. They, however, start from the assumption that the basic macromolecules (nucleic acids and proteins) already exist in the environment, and therefore they do not explain how these molecules were formed. Furthermore, it is not known how these molecules have accumulated selectively within the membranes.
According to J. B. Bernal (cited work), the compartments were, instead, cavities and intercrystalline spaces within clay granules. In these compartments constantly in contact with the outer environment, could accumulate and interact the molecules necessary for the origin of the proto-organism. In addition, macromolecules dispersion in the outer environment or their demolition due to ultraviolet rays could be avoided. It should be noted that the possibility of accumulation of simple molecules and polymer syntheses within the clay has been widely demonstrated in various researches. This hypothesis on the compartments, shared by several researchers, seems more credible because it does not start from any assumption. The clay compartments exist today and existed in the prebiotic era. Now, if the proto-organism originated within clay cavities, it was not necessary in that first phase to have the proto-cell membrane. If the latter element was absent, the genes and proteins necessary for its replication were absent, the genome had to be reduced by some units.
2) In all living organisms a nucleic acid, DNA, has the function of archiving genetic information. DNA portions, the genes, are transcribed in messenger nucleic acid, mRNA. It is the mRNA that translates information into proteins. But has the DNA always existed?
Almost all scientists today agree with what Mario Ageno wrote at the beginning of the 1980s in the chapter entitled “Dai precursori al proto-organismo” (quoted work). He made a depth analysis on the subject and wrote: «It is conceivable that at the beginning the transcription did not exist. A single nucleic acid with a single propeller could simultaneously carry out the information archive function and actively intervene in synthesis operations». If the DNA was absent, the group of proteins necessary for its replication was absent. The genome of the proto-organism get much more simpler, at least by a dozen units.
3) Protein synthesis is a fairly complex process. It needs a messenger RNA, transport, RNA (adapters), a Ribosome, enzymes for the synthesis of the protein polypeptide. Could such a system be present in the proto-organism?
Ageno still writes: «It is conceivable, indeed practically certain, that the ribosome, if it existed, was initially different from now, reducing itself only to the nucleic component. But it is also possible that at the beginning the ribosome did not exist and the synthesis occurred by interaction between the RNA and the loaded adapters with their amino acids. It is likely that the adapters, possibly simpler than the current ones, existed from the beginning. Otherwise, it would be necessary to postulate specific direct interactions between nucleotide triplets and amino acid, [...]». Now, since here it is believed that in prebiotic period a direct and specific interaction between the trinucleotide and the amino acid existed, it means even tRNA were absent.
The ribosome of the present bacteria contains about 50 proteins. The tRNA need at least 20 specific enzymes to bind each amino acid to each tRNA. Once aligned the tRNA, several other enzymes are needed to bind the amino acids in the polypeptide. If all this complex system did not exist, even if the tRNA were absent, the proteins and therefore the genes necessary for their synthesis were also absent, the genome of the proto-organism is therefore considerably reduced.
Ultimately, an approximate calculation of the above points leads to the conclusion that proto-organism could begin their existence with a genome of about 100 genes and as many proteins.
We then begin from the hypothesis that the proto-organism contained a genome of RNA of about 100 genes.
As we have already mentioned, around 1970, the hypothesis that proteins were made up of domains, that is, a sequence of amino acids that is preserved during evolution, has been advanced. In 1974, Rossman identified a domain of about 70 amino acids present in many enzymes and suggested that this domain was also of prebiotic origin (cited article). But many scientists today believe that the domains in prebiotic era they were smaller and mainly consisting of ꭤ-helices of 30-40 amino acids. For Mike Williamson in "Come funzionano le proteine" 2013, the domains were even smaller and made up of ꭤ-helices of 20 amino acids.
But let us take for domains an average of 30 amino acids.
Now, for every gene a protein, if the 100 genes encode for 100 proteins of 30 amino acids on average, what was the genome size of the proto-organism?
According to the genetic code, a triplets (i.e. a trinucleotide) encode an amino acid (3:1), so to specify 100 proteins of 30 amino acids, meaning 3000 amino acids, a 9,000-nucleotide genome is required. We take as an example the image of one of the four nucleotides: adenosine-5-phosphate
Therefore, we can conclude as follows:
The proto-organism originated inside a clay cavity, where the fundamental substances for the origin of life accumulated and interacted. It was in constant contact with the environment for the supply of the substances necessary for its chemical evolution. DNA did not yet exist as archive of genetic information, nor was there a single RNA genome to perform the double function of archive of genetic information and protein synthesis and tRNAs were not present for the transport of amino acids. The proto-organism had to be composed of single RNA genes, about 100, initially completely independent, and about 100 proteins. Protein and RNA constituents, and small organic molecules from the outside environment, had to be present inside the proto-organism. RNA synthesis and protein synthesis took place by direct interaction between amino acids of a protein and trinucleotides, and between RNA trinucleotides and amino acid respectively.
Once defined the proto-organism, two questions arise:
How did the proto-organism originate from inanimate matter?
How did the passage from the proto-organism to the cell occur?
9.2 Origin of the proto-organism: The protein entity and chemical-physical significance of homeostasis
We have already described the origin of the elements and the origin, from inanimate matter, of fundamental substances for the origin of life, in particular amino acids, formaldehyde (HCHO) and hydrogen cyanide (HCN). Furthermore, it has also been widely described how the clay, according to the Bernal hypothesis, was able to select, accumulate and protect these fundamental substances. The clays therefore functioned as a physical regulating agent and a fundamental role must have played colloidal silica. In addition, we have also described how within these compartments a small-scale thermodynamics comes into play, whereby the formation of the polypeptide becomes a spontaneous process. Then the Left amino acids accumulated inside the double electrical layer of the colloidal silica, within the clay micro-cavity, give rise to polypeptide chains (primary structure) wrapped around the colloidal silica.
However, the colloidal silica particles have a very short life. If a colloidal silica particle, on which a polypeptide was synthesized on its surface, meets other colloidal silica particles, amorphous silica will form. The electrical interactions between colloidal silica particles are so strong that they deform each other.
The polypeptide, no longer finding the original electrical interactions, detaches from the surface and goes into solution inside the cavity. Since the colloidal silica was helicoidally, necessarily helicoidally will be the course of the polypeptides. These structures are, in aqueous solution, thermodynamically unstable and, due to thermal agitation and impacts with water molecules, they should have decompose into amino acids and fall into energy state 2.
As we saw, the decomposition of polypeptides into amino acids while being thermodynamically possible is kinetically impossible. Water molecules do not have enough energy, at room temperature, to break all the polypeptide bonds. The decomposition takes place but at a very low speed; an energy barrier therefore prevents rapid decomposition. However, the polypeptides contain positive charges and negative charges inside them.
Each polypeptide, before being slowly decomposed by water into individual amino acids and precipitated in State 2 as required by thermodynamics, spontaneously establishes links between the positive and negative charges that stabilize the helical structure (secondary structure). The formation of the stable and ordered structure, called α-helix, frees energy that increases universal entropy.
The α-helix is therefore located in a pit of Energy, State 1.
It is probable, however, that the amino acid composition of the α-helices was different in different areas of the planet as a consequence of local chemical-physical conditions. Consequently, one can only conclude that the polypeptides, produced by ordinary chemical physical forces and by local chemical physical conditions, in the form of α-helix had to be found, in prebiotic times, in great abundance on the whole surface of the planet, in every cavity, in every pore, in every niche of clayey masses.
As highlighted by Duranti Marcello in "Introduzione allo studio delle proteine" 2015: «Some α-helices contain hydrophobic portions of the cylinder, this gives rise to interactions between hydrophobic amino acids which give rise to super-secondary structures that are the first step towards the tertiary structures of protein».
What has been described up to now, that is the origin of the polypeptides, is in principle accompanied by experimental data. The path followed by the polypeptides towards the origin of the proto organism, and so towards the origin of life, is a true mystery for science. Having no experimental data available, to understand the origin of the proto-organism, we can proceed, with an effort of logic and imagination, only through a credible narrative.
So, let us imagine a niche, a micro-cavity inside a clay mass, where a few hundred α-helices have accumulated. Some α-helices gave rise to super secondary structures and subsequently to tertiary structures. The tertiary or globular structures contain within them hydrophobic groups and, on their surface, hydrophilic groups with residual electric charges. Therefore, tertiary and α-helical structures were certainly surrounded by clusters of water to form a complex interactive protein system.
If we want to make an extreme summary, we would imagine that residues with negative charges are found on the surface of a globular polypeptide, they would be wrapped around a cloud of water molecules with Hδ+ oriented towards the negative.
In the contact zone between the two water aggregates, water will disposed itself as to minimize the electrostatic repulsion.
Within the cavity, all the components of the interactive system would have to be included, therefore, within an ordered macrostructure, “almost crystalline", of water and the interactive protein system would assume the appearance of a gel.
To this macrostructure, we can extend the concept expressed by Peter W. Atkins with reference to the α-helix reported: The ordered disposition of all the molecules of this gel macrostructure is preferred over an irregular cluster because that corresponds to the situation of greater chaos of the universe. The macrostructure is certainly endowed with a minor chaos due to the ordered arrangement, but the universal chaos is greater because of the energy that is released when the strong hydrogen bonds are formed. Like the stone on the hill that sinks more and more after each storm, so does the macrostructure fell into an energy pit, represented by State 1, gaining a great chemical stability.
This interactive system between protein molecules operates, therefore, within the second principle of thermodynamics, by which order generates chaos, the formation of complex structures to produce entropy.
Within this gel structure the components of the system communicated through the electromagnetic force generated by the surface potentials. Now it is evident that if from the external environment one or more molecules rich in energy come into contact with the gel of the micro cavity, the nearby polypeptide begins to destabilize by changing its shape. This change induces the water that surrounds the polypeptide to take another arrangement. This new arrangement will force all water molecules of the gel to re-orientate electrically, passing the information to all the macromolecules of the system that, in large or small size, will be subject to changes their shape. The energy accumulated by a single polypeptide is discharged and shared by the whole complex interactive system. The new arrangement of all the other macromolecules making up the gel will send a feedback signal that will indicate to the first macromolecule whether to reject or absorb, to synthesize and what to synthesize. Only systems that succeed in developing a communication system that minimizes errors will flourish. The complex interactive system is now an entity and presents a rudimentary homeostasis that is the ability to maintain a more or less constant uniform chemical equilibrium in a changing environment.
However, the homeostasis so defined is only an idea, a concept.
How can we physically represent this entity, and what really is homeostasis?
The formation of molecules from atoms always involves electrical charges. Around the molecule of a compound, we must imagine an electromagnetic field with its own specific energy content, which differs from other compounds. This electromagnetic field defines the properties of the compound. For example, in a droplet or a glass of water, the electromagnetic field surrounding all the molecules defines the liquid state of the water at room temperature. The electromagnetic field that surrounds the molecules of an amino acid confers its solubility in water. When tens of amino acids bind to form an enzymatic protein, the electromagnetic field around its molecule not only defines the intrinsic properties such as solubility, but also gives a function: the enzymatic function that is the enzyme through its electromagnetic field recognizes and splits or binds specific molecules. When hundreds of enzymes are surrounded by clusters of water they give rise to an interactive protein system, precipitated in a pit of energy and therefore very stable, the electromagnetic fields of all the molecules inside it give rise to an electromagnetic field around the whole system that organizes and controls the system itself and identifies it as an entity. Now, the electromagnetic field of the protein entity certainly generates properties and functions, which to a macroscopic level manifests itself as homeostasis. However, if the entity is under the controls of the electromagnetic field, we can to define homeostasis: the response of the electromagnetic field around and inside the entity to changes in the external environment and the internal medium. However, homeostasis is an emergency associated with a complex interactive system precipitated in an energy pit and therefore in chemical equilibrium. Homeostasis, through chemical reactions and feedback cycles, tends to preserve this balance. Since this entity presents homeostasis, we can identify it as a primitive protein cytoplasm.
The homeostasis defined as the response of the entity's electromagnetic field to changes in the external environment and the internal medium is no longer a concept, but takes on a chemical physical meaning.
The second fundamental step towards the origin of the proto-organism is the formation of short RNA molecules.
But how did the formation of RNA occur?
The RNA consists of nucleotides. The latter are formed by the link between a phosphate group (H2PO4-), the D-Ribose, belonging to the family of sugars, and one of the four nucleobases: Adenine and Guanine, belonging to the Purine family; Uracil and Cytosine belonging to the Pyrimidine family.
The problem that arises is understanding if these constituents were present in the prebiotic era.
The phosphate group is found in nature in the form of volcanic minerals, apatite (calcium phosphates, Ca5 (PO4)3[F, OH, Cl].) which, in the form of very small crystals, are scattered over the entire surface of the planet. We have no element that could indicate that the situation in the prebiotic era was different from now. The solubility of apatite is very low, of the order 30 µg / L (µg / L = millionths of grams per litre). Therefore, even if in small quantities, the phosphate group was present in the prebiotic era.
In relation to the nucleobases, in 1961 Juan Orò, one of the most engaged chemists in prebiotic chemistry research, succeeded in synthesising Adenine by heating a high concentration of (HCN) hydrocyanic acid at 70 ° C in the presence of ammonia (NH3). In this experiment several organic substances were obtained and among these Adenine. Later, Orò was able to synthesize Guanine too. Regarding these experiments C. Ponnamperuma in "Origine della vita", 1984 comments: «[...] the concentrations used by Orò were far too high to correspond to a prebiotic situation. If the experimental conditions were really similar to the prebiotic ones, for example, if lower concentrations had been used, then these reactions would be of great help in understanding the origin of the purines in the conditions present in the prebiotic phase of the Earth».
Unfortunately, after these experiments and for over 50 years there are no significant experiments.
The reason is probably to be found in the arrogance of the supporters of the "RNA World" who have transformed a hypothesis into a confirmed model, considering the research on nucleic acid constituents superfluous.
After this long period, it seemed that research on the origin of the nucleic acid constituents had fallen into oblivion, until two Italian scientists, Ernesto Di Mauro and Raffaele Saladino, reopened the game.
Their experiments described in the essay "Dal Big Bang alla cellula madre: l’origine della vita" 2016, are of considerable interest. First of all because, instead of using HCN (Hydrogen cyanide) which is a gas, they obtained nucleobases using the HCONH2 (Formamide) which has a boiling point above 200°C, and which was certainly present in the prebiotic era because it was produced by reaction between HCN and H2O. Moreover, these experiments take place using clay or minerals certainly present in the prebiotic era. These experiments are fully part of the Bernal theory. In fact, Bernal hypothesized that clay could function as a regulatory principle to select, accumulate, protect and catalyze the fundamental substances for the origin of life. Therefore, we find ourselves, in prebiotic times, the necessary bases for the nucleic acid right inside clayey masses, where the primitive protein cytoplasm originates.
In relation to Ribose it should be pointed out that its molecule, like the molecules of amino acids, has a Right and Left shape, a mirror image of the other, but only Right is used in nucleic acids. Ribose, like Arabinose, Xylose and Lisose, is a pentamer of formaldehyde (HCHO), in the sense that it is made of 5 molecules of formaldehyde, but is, in an aqueous solution, an unstable compound.
Around 1880 A. Butlerov treated formaldehyde in a strongly basic environment, he succeeded in synthesizing Ribose, a reaction known as the reaction of the formose. This reaction does not work in prebiotic conditions, besides together with the Ribose a mixture of other sugars are formed, including the other three pentamers, which would have hampered the formation of the nucleic acid. In the absence of valid research, in 1994 L. Orgel on Le Scienze, "L’origine della vita sulla terra" wrote: «First of all, in the absence of enzymes, it is problematic to synthesize Ribose in adequate quantities and with a sufficient degree of purity».
In 2008 in Christian De Duve (quoted work) takes into consideration the researches of Prieur (2001) and Ricardo (2004) who using the borates managed to stabilize the Ribose and limit to the formation of other sugars. Ricardo, in "Planetary Organic Chemistry and the Origins of Biomolecules" 2015, describes in detail the mechanism of reactions and the function of boron, but also reports Hazen’s criticisms which defines boron as an "exotic" element for prebiotic chemistry. Christian De Duve also reports a work by Ricardo e al. 2004, which obtained the four pentose (both Right and Left) by making glyceraldehyde to react with glycol aldehyde.
Also Di Mauro and Saladino pickup on the works of Prieur and Ricardo but they add that they have achieved similar results using Zirconate. Now, the fact is that the Zirconate are anything but "exotic", they even if in small quantities are distributed across the whole surface of the planet and mainly in sedimentary and metamorphic rocks. The clay, in relation to the composition, distinguishes itself as kaolinite, beidellite and montmorillonite. In beidellite clay, more than the average Zirconate of the planet was found. Therefore, we find ourselves once again within the Bernal theory. So, in prebiotic times, besides a primitive protein cytoplasm contained in the clay cavities, it is probable that dozens of sugars both Right and Left and dozens of nucleobases, were spread inside clayey masses. From this mixture of nucleobases and sugars, diffused in the clay masses, only four nucleobases, Adenine, Cytosine, Guanine and Uracil and only one sugar the D-Ribose were co-opted within the rudimentary protein cytoplasm.
Why these and not the others, what constraint imposed this selection?
To select these compounds must have been the homeostasis of the primitive protein cytoplasm.
The homeostasis responsible for maintaining chemical balance within the protein cytoplasm, allows the diffusion of only substances that maintain this equilibrium.
As we have already mentioned, homeostasis, is the response of the electromagnetic field of the entity with respect to changes in the external environment and the internal medium. So, let us try to give a physical explanation to the events.
Let us imagine having a glass of water and adding sugar. We can simplistically say that the electromagnetic field around the sugar molecules is compatible with that of the water molecules and therefore the sugar dissolves in water. If instead we put a drop of oil in the glass, the electromagnetic field around the molecule of the oil is not compatible with that of water, the oil does not mix with water and collects on its surface. Now, let us imagine how the electromagnetic field could have been like, generated by hundreds of α helices, around our protein entity. These α-helices were made up of L-amino acids and all had a right-handed trend. The electromagnetic field around the protein entity had to comply with the right-hand helical trend of the α-helices and therefore had to necessarily be right-handed. Now, since the molecules of D-Ribose and L-Ribose are one image of the other, their electric field must also be the mirror image of the other, which are, right-handed and left-handed. Then, when in the prebiotic era D-Ribose molecules and L-Ribose molecules tried to spread inside the cavity where there was a right-handed α-helix entity, the homeostasis would co-opt the D-Ribose, because complementary to the electric field of the entity, while its mirror image, L-Ribose would be rejected.
In addition to the Ribose, in the clay masses there were certainly other sugars similar to Ribose, Right and Left too, such as the Arabinose. D-Arabinose, like D-Ribose, was certainly complementary to the electric field of the protein entity.
But why was D-Ribose chosen and not D-Arabinose?
Simplistically, we can conclude that Adenine, Cytosine, Guanine, Uracil and D-Ribose are soluble in the protein entity while no other nucleobases and sugars are soluble.
We identified the protein entity that presents homeostasis as a primitive protein cytoplasm.
But the rudimentary protein cytoplasm is a set of enzymes. Within the entity, these enzymes, using the little phosphate available in the solution, bind D-Ribose correctly with one of the nucleobases and phosphates creating the nucleotides. The compounds that gave rise to the nucleotides could have bonded in many different ways giving rise to a large number of different nucleotides of no use for life. The synthesis of the right nucleotides was a real chemical engineering work possible only through a specific enzymatic process. Finally, other enzymes bind three nucleotides in the right way giving trinucleotides. As we have hypothesized, during the prebiotic era there must have been a direct interaction between a trinucleotide and a specific amino acid, a chemical physical system of recognition and complementarity. Now, when the trinucleotides spread within the entity and meet an α-helix, each trinucleotide pairs with the specific amino acid of the α-helix. When each amino acid of the α-helix is superimposed by the specific trinucleotide, it will be the enzymatic action of the α-helix to bind the trinucleotides giving rise to RNA, ribonucleic acid. Since the RNA was synthesized by a helical enzyme, the α-helix, it turns out to have a helical structure. If there were a hundred different α-helices in the cavity, they would give rise to a hundred different RNA. The nucleic acids replace the silica and with the amino acids in solution, they can synthesize the enzymes that for various causes were decomposed. To use the Cairns-Smith metaphor in “Sette indizi sull’origine della vita” 1986: the armature, the silica, generated an arch, the α-helix, which in turn generated an armature, RNA, which definitively replaces the former. Nucleotides synthesis (ribose + nitrogen base + phosphate group), trinucleotides synthesis and RNA synthesis all occur in the non-aqueous microenvironment of the surface of the enzymes. These conditions allow the enzyme an extraordinary reactivity, different from those in an aqueous environment. Furthermore, in all these synthesis reactions, water molecules are released, which will increase the universal chaos. Order is created by increasing entropy: Chaos from order.
Homeostasis maintains balance, but with the appearance of the RNA the entity is expanded, giving rise to a cytoplasm containing an interactive nucleic acid-enzyme system that sinks even further into the energy pit. Homeostasis maintains equilibrium through the electromagnetic field around and within the entity.
The new entity, which is the cytoplasm nucleic acids-enzymes, is the proto-organism.
In conclusion, since the second principle of thermodynamics is not able to follow the path of maximum chaos represented by the energy level of state 2, it follows the path of possible chaos and digs a parallel energy ditch represented by state 1.
Along this path, through various energy jumps, order generates chaos until it causes the origin of the proto-organism. The proto-organism has always been considered as a system far from the thermodynamic equilibrium described by the state 2. In reality the proto-organism is a system far from the maximum chaos but is in equilibrium with the possible chaos and therefore under the control of the 2nd principle of thermodynamics.
So, we are faced with this scenario.
In billions of billions of cavities, niches and inter-crystalline spaces of an unspecified but enormous number of clay masses, an infinite number of polypeptides are synthesised which give rise to an immense number of primitive protein cytoplasm. Nucleobases and D-Ribose molecules contained in the clay masses are co-opted within the protein cytoplasm. Helical polypeptides that synthesize the nucleic acid. The interaction between nucleic acids and enzymes gives rise to an endless number of proto-organisms.
But the proto-organisms formed near volcanic areas must surely have been different. These proto-organisms had to contain proteins that had originated in those areas and therefore future cells develop different metabolic pathways.
We have yet to clarify, and we will do it later, because life is unitary.
















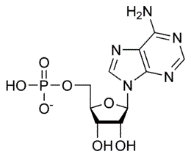







Nessun commento:
Posta un commento