Hence, for the
peculiarity of their atomic structures, the biogenicelements are the only ones, which through their compounds are adapted to
carry out, in living organisms, the numerous biological functions. We know that
between these compounds the most important are the nucleic acids, DNA and RNA, and the proteins. Nucleic acids and proteins are
interdependent, in the sense that nucleic acids contain instructions for the
assembly of proteins but it is the proteins that operate the synthesis of
nucleic acids.
Oparin-Haldane's
theory postulated that life originated in a primordial soup. However, it is
extremely unlikely that these macromolecules originated simultaneously,
independently and that by meeting randomly they began to interact. The problem
then arises: did proteins (metabolism) or nucleic acids (the genetic apparatus)
arise first? The experiments conducted by Miller and other researchers in the
`50 and` 60 have shown that the amino acids,
constituting proteins, are easy to synthesize in a prebiotic environment, while
the constituents of nucleic acids, i.e. sugars
and nucleobases, are very difficult to
synthesize. So it was logical to think that proteins first appeared. When the
failure of protein synthesis in the prebiotic broth became evident in the late
1960s, researchers began looking with interest at nucleic acids.
Between the
two nucleic acids the RNA has given origin to the theory of the “RNA World”; it was preferred because it is less
complex, it participates in the assembly of proteins and in some viruses it
replaces DNA as genetic material. The "RNA World" theory postulates
that life originated through the appearance of self-replicating RNA molecules.
But, were the
constituents of RNA present in the prebiotic era?
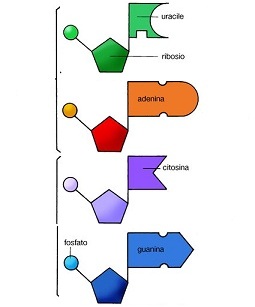
The RNA is a large molecule whose unities constitutive are four nucleotides.
Certainly for
the formation of self-replicating RNA molecules it is necessary first of all
the presence, in prebiotic times, of all the constituents listed above and,
secondly, understanding how they linked to form RNA.
The phosphate
group which, even if in small quantities, is diffused on the whole surface of
the planet, and there is no reason to believe that it was not present even in
the prebiotic era.
In reference to ribose, Ponnamperuma in "Origin of life", 1984 states:
«In contrast with the progress obtained in the field of primordial chemistry of the amino acids, the origin of another group of substances just as important biologically the monosaccharides (sugars), is even now fairly uncertain. Already in 1861 A. Butelow had shown that formaldehyde dissolved in water goes towards a process of condensation in an alkaline ambiance, giving place to a mixture of sugars. […]. There are for this reason various difficulties in accepting the hypothesis that formaldehyde could have been used as a precursor of the monosaccharides. Horowitz and Miller have made it clear that the high concentrations of formaldehyde used in some experiments do not reproduce in a realistic way the conditions of the primitive Earth. Objections have also been made to the use of very basic solutions. P. H. Abelson sustains that the concentration of ammonia free in the seas and in the atmosphere was not ever so elevated, and that a strong alkaline ocean never existed. Furthermore, formaldehyde polymerizes fairly rapidly giving paraformaldehyde, and so can go a certain distance from the ambiance […]
In 1994 in Le scienze, "The origin of life on earth", referring to ribose L. Orgelwrites: "It is also possible that some non-enzymatic reactions capable of leading to the synthesis of pure ribonucleotides have not yet been identified". He mentions Albert Eschenmoser who managed to limit the number of different sugars produced in the synthesis of ribose from formaldehyde in the presence of borates and under particular conditions, he also obtained a phosphorylated ribose derivative.
In 2004, in
Prebiotic Chemistry and the Origin of the RNA World, Orgel resumed the
well-known Butlerow reaction of 150 years ago. He writes that if this reaction
could be directed towards the synthesis of ribose it would be ideal for the
synthesis of the sugar component of the nucleotides. Cites Zubey (1998, 2001)
who repeated the reaction in the presence of lead. It also highlights how
calcium borate stabilizes pentoses (Ricardo et al., 2004). He admits that all
these reactions take place at too high pH and with high concentrations of
reagents. Finally he concludes that some progress has been made, but that there
are still a number of obstacles to the synthesis of significant and pure
quantities of ribose.
And with
reference to the nucleobases in the same essay C. Ponnamperuma adds:
«The purines were synthesized for the first time in conditions which simulated those of the primitive earth by Orò(v. Studies in …1963), who demonstrated that one could obtain the synthesis of Adenine by a concentrated solution of ammonium cyanide. In its broad lines the reaction can be represented as 5 molecules of hydrogencyanide that in the presence of ammonia give place to Adenine. This synthesis has be confirmed by Lowe […]. Afterwards, Orò succeeded in synthesizing Guanine and Xanthine by bringing to a temperature of 100-140°C a solution with water of amminoimidazolcarbossiammide. The rendering was of 1,5% for both the purines. It is possible that this was one of the ways in which the synthesis of purines on the primitive Earth took place, but the concentrations used by Orò were very much too high to correspond to a prebiotic situation. If the experimental concentrations had been really like those prebiotic, if for example lower concentrations had been used, then these reactions would be a great help to the understanding of the origin of the purines in the condition present in the prebiotic phase of the Earth. In spite of the efforts made, it was not possible to identify with certainty purines and pyrimidines among the final products in experiments that used electric charge. As a great quantity of hydrocyanic acid is formed, it is difficult to understand how the purines could be absent. Very little has been done in the region of the synthesis of the pyrimidines. Fox and Harada (v., 1961) have demonstrated that Uracil can be obtained by the heating of malic acid and urea. Whereas urea is easily formed in the experiments, which simulate the primitive Earth, there does not exist any indication of the presence of malic acid. Fox and Harada […] and others (v., Formation of…,1963) have obtained also the synthesis of adenine through electrons flux on methane, ammonia and water […]. The greatest part of the radiations produced by radioactive sources is absorbed by solids, and as the earth’s crust has a thickness of about 30 km, this type of radiation could have had no role in the synthesis of organic material in the primitive oceans».
Hence Ponnamperuma affirms, in 1984 that these
reactions are not of great help to the understanding of the synthesis of the
nucleobases in the prebiotic phase. These however do not offer any indication of
the presence of nucleobases in the prebiotic era.
In 1995 Christian De
Duve, although a sustainer of the “RNA World” in “Polvere Vitale” affirms:« […]
chemists have had a certain success in the production of the five organic components
of the RNA, but with scarce rendering and in conditions at the same time very
different from a prebiotic scenery and different for every substance. If one
wants to combine the components in the right way, one comes across other
problems, of such magnitude that no one has ever tried to do it in a prebiotic
contest».
In 2004 Orgel, “Prebiotic
Chemistry and the origin of the RNA World”, tries to unify the scene at least
for the four nucleobases.
He shows how Adenine
can be obtained from a eutectic solution, very
concentrated, of NH3 and HCN at the temperature of -23,4 °C, that is
the synthesis of Orò revisited. Orgel points out that among the products of
this reaction, traces of Guanine were found.
Orgel observes how the
Uracil can be obtained by the hydrolysis of Cytosine.
For the synthesis of Cytosine
he takes first into consideration the reaction between cyan acetic aldehyde and
urea concentrated, but he concludes that, in the prebiotic era, such a reaction
is not plausible. However it seems to him more plausible the reaction of a
eutectic solution of cyan acetylene with cyanide because it could proceed in
parallel with the synthesis of adenine also in eutectic condition.
On paper under the
word “eutectic” the synthesis of the four nucleobases were done with the same
logic. But temperature and concentrations
indicated by Orgel are completely outside any prebiotic context. It is overmore
necessary to keep in mind that these synthesis also cause the formation of many
nucleobases without interest, even obstacles for the origin of life.
To be short, in 2004,
that is after a half century of tentatives by the best chemists in the world,
we can conclude that, in the prebiotic phase, no chemical process has produced
the four nucleobases and Rybose.
Furthermore, the
following problems arise for the origin of RNA molecules:
In the pentoses, three
carbon atoms are asymmetric and thus we have three chiralcentres. This implies that the number of possible molecules (stereoisomers) is equal to 23 that is 8, of
which four D (right) and four L (left) and among these the Ribose. It is true
that every couple D-L has chemical-physic characteristics slightly different
with respect to others, but from an energy point of view in a prebiotic phase,
they all have the same probability to being synthesized. Now only for the
spontaneous assemblage a nucleotide illustrated below,
the following passages
are necessary:
1) Origin and
separation of pentose from all other sugars
2) The separation of
the four pentose D (right) from the L to avoid crossed reactions.
3) The separation of
the D-Ribose from the other three sugars D to avoid superposed reactions.
4) Origin and separation
of the four nucleobases necessary for the RNA from all the others.
5) A particular
orientation, to obtain the four nucleotides, of all the nucleobases on OH of
C-1, and to avoid the reaction with OH from C-2, C-3, C-5, (as illustrated
above).
6) A particular
orientation of the phosphate group on OH of C-5 to the end of avoiding the
reaction with the OH from C-2, C-3 (as illustrated above)
7) The orientation of
all the nucleobases in the position β (that in upwards as in the figure).
And finally for RNA:
8) The reaction of
phosphate of the nucleotides with OH in the position C-3 of other nucleotides,
with the exclusion of the position C-2, to give origin to the RNA.
In over 50 years of
research, there have not been and there is not even now a chemist who has
succeeded in corroding points 1, 2, 3, 4. And there has not been and there is
not even now a biologist who can give an explanation to points 5, 6, 7, 8,
without the intervention of specific enzymes.
It is important once
again to underline that all the points listed above concern the original
conception of the "RNA World", that is: the origin of life through
the spontaneous appearance of self-replicating molecules.
Starting from 1967,
experiments were conducted by Sol Spiegelman, Manfred Sumper and others using
artificially activated nucleotides and Qß replicase (a very complex biological
enzyme). From these experiments, RNA molecules ex novo of different composition and dependent on the experimental
conditions were obtained. Concerning these experiments Manfred Eigen and others
in "The origin of genetic information" The Sciences 1981 states:
"What is important here is what these experiments reveal about Darwinian
processes. Natural selection and evolution, which are consequences of
self-replication, operate at the level of molecules as well as at the level of
cells or species ".
It was not yet clear
how the first RNA molecules appeared, but Darwinian processes begin to enter
the "RNA World".
This idea of Eigen referred to RNA macromolecules is gradually extended
to the molecules that make up RNA. So Christian De Duve in the essay "To
the origins of life" 2008, with reference to the synthesis of nucleotides,
taking note that, in the prebiotic era, D-Ribose was certainly in the presence
of other sugars, also of opposite chirality, and that in addition to the bases
constituting the RNA were certainly present other bases, he writes: «It seems
more probable that life began with a Gemish,
a mixture of many different molecules of similar structure and that those found
in organisms living today emerged later selection».
This key notion was
taken up in 2016 by two Italian scientists Ernesto Di Mauro and Raffaele
Saladino in an essay: "From the Big Bang to the mother cell". In it
the authors state that the solution has been Darwinian since the very
beginning: the survival of the fittest. "Without selection there is no
life, especially at the molecular level".
With De Duve, Di Mauro
and Saladino (and obviously the contribution of other researchers), Darwinian
processes are extended to the constituents of RNA and a new conception of the
"RNA World" is definitively born: the origin of life through the
appearance of self-replicating molecules by natural selection.
If this is the key
notion, if the molecular selection already begins at the level of the
constituents, then it is no longer important if, in prebiotic times, other
sugars were present in addition to D-Ribose and if other bases were present in
addition to the canonical bases, and their concentrations are not important
either because the most suitable, not the most abundant, will survive. And the
thought of A. Graham Cairns-Smith is passed according to which, thinking about
the role of large times and large spaces, to generate structures, can lead
astray. For scholars of the "RNA World", the fact that the
constituents can be formed even if in the presence of different catalysts and
in extreme space and energy conditions, however, demonstrates the possibility
of their synthesis in the prebiotic era.
It is then, we follow
the essay by Di Mauro and Saladino where the results of their research are also
exhibited and which is the only essay in dissemination today that makes us
understand comprehensively the state of the art of the world at RNA.
Instead of using high
concentrations of hydrogen cyanide (HCN) which is a gas, as Orò worked to
obtain nucleobases, the authors used formamide
(HCONH2) which has a boiling point of 210 ° C.
The formamide heated
to 110 ° C in the presence of clay produced Adenine, Cytosine and Uracil, three
of the five nucleobases. In a second experiment, the clay was replaced with
Titanium oxide (TiO2) obtaining Adenine, Cytosine and Thymine. Although the formation
of the fifth nucleobase of Guanina is lacking in the presence of terrestrial
minerals, and it cannot be excluded that it can be soon identified, the results
are of considerable interest. First of all, formamide was certainly present in
the prebiotic era; furthermore the temperature of 110 ° C on the mainland is
locally plausible; finally the presence of clay and titanium oxide widely
present on the planet's surface bring us back fully to Bernal's theory. This
theory, in fact, postulates that clay had a decisive role in selecting,
accumulating and catalyzing the formation of complex molecules. We can conclude
that the presence of the nucleic acid constituents in the prebiotic era is
plausible.
It is important to
note that cellular metabolism intermediates were obtained in these experiments.
As reported by Di
Mauro and Saladino, in 1992 Eschenmoser defined a biological process that
mimics a prebiotic reaction (used in practice as a mold) as chemomimetic. In
short, as the authors write: «The complex primary and secondary metabolic
systems that we observe today operating in the cell would, on the basis of this
definition, be the highly specialized version of the first prebiotic reactions
around which molecular evolution would have built enzymes and systems
increasingly complex structures to optimize each process in terms of energy and
selectivity. It follows that, if chemomimesis exists and has controlled
molecular evolution, a given prebiotic reaction should produce not only the
fundamental biological molecules, selecting them from the thousands of possible
structural variants (isomers), but also the main intermediates for their
achievement , in perfect agreement with those still functional in the cell ».
Ribose molecule is
often represented in linear form:
CHO
I
H -*COH
I
H -*COH
I
H -*COH
I
CH2OH
In reality, Ribose has
a cyclical form:
When you move from the
linear structure to the cyclic structure, there is the formation of α and ß
anomers, that is, the OH can be below or above and reacting to give rise to
different compounds. The α position is energetically preferred but in the nucleotides
the four bases are all in the ß position.
Why this recall?
Because during the
thermal condensation in the reaction between formamide and TiO2, formulated
derivatives of adenine were obtained with the nucleic base bound to short C-1
sugar molecules already in the ß position.
This intermediate of
the genetic apparatus, reacting with HCHO, could lead to the formation of a
nucleoside (nucleotide without the phosphate group).
In experiments, also
conducted by other researchers with formamide in the presence of borates or
zirconates, sugars and nitrogen bases have been identified alongside
intermediates of the current cellular metabolism such as pyruvic acid, lactic acid
and α-amino acids.
Metabolism
intermediates, in particular the Krebs cycle,
were identified with TiO2 and formamide irradiated with UV.
The discovery of
metabolic and genetic apparatus intermediates has expanded the RNA world
program towards the unification of genetic vision with metabolic vision. That
is, as Di Mauro and Saladino report, the search for «A prebiotic pathway
capable of simultaneously producing intermediates of the genetic apparatus and
components of the metabolism».
In the new conception
of the "RNA World", a contribution of substances coming from space is
also taken into consideration, in particular on the granules of the huge clouds
of interstellar dust. Space dust analogues were produced in the laboratory and
in the presence of formamide, they gave rise to nucleobases.
The contribution of
meteorites in the presence of formamide was also studied, the presence of
intermediates of both the metabolic and genetic apparatus was observed.
A high energy laser
source was used to produce high temperatures and to study the impact of
meteorites on the earth's surface in the presence of formamide. The presence of
the five nitrogenous bases and glycine has been observed.
To simulate the
contribution of the solar wind, high-energy
protons, meteor dust and formamide were used at a temperature of -35 ° C. In
these experiments the nucleic bases and for the first time the four nucleosides were obtained.
Phosphorylation
reactions of nucleosides in the presence of formamide and phosphoric minerals
have highlighted the possibility of nucleotide formation.
At this point, the
formation of nucleotide chains for the formation of RNA, according to the
authors, lies in the nature of things, in the strength of their chemical
nature, in the principle of chemical affinity. That is, he seems to understand,
a chemical need that could not fail to happen.
Replication is a
chemically simple phenomenon due to chemical affinity which, in the presence of
the right complementary monomers, in the right combinations and at the right
temperature, could have happened by chance.
Variability is
inherent in the chemical nature of the molecules and in the reactive properties
of RNA.
For the RNA-Protein
interaction you imagine the spontaneous formation of di- or tri-peptides that
initially favoured the replication of RNA by entering through continuous
functional verification into a winning evolutionary system.
For the origin of the
genetic code, the possibility of the "frozen accident" is privileged;
that is, other codes were possible and perhaps even existed, but once this
route was taken it was not possible to go back.
While considering the
compartmentalization in phospholipid bags as fundamental, in the initial phase,
the formamide-heat-concentration-catalysis route is the most suitable
environment in the "geothermal fields".
Hoping that the
necessary synthesis has not misunderstood some parts, we close here with the
results and the new vision of the "RNA World", that is, a world where
necessity, chance and evolution are intertwined.
As can be seen from
the articles in this Blog, we privilege an exclusively deterministic way and
that to appear first were the proteins.
Further studies in the
future will perhaps tell us which way is the most plausible or if the two ways
will meet one day, for the moment it is right to wish all the scientists
involved in this work good work.
Giovanni Occhipinti